 New Dosage Form (Topical) New Dosage Form (Topical)
Utilizing the proprietary formulation designs and production techniques, Andros Pharma’s new dosage form drugs enhance drug permeation across stratum corneum, reduce skin irritation, prolong drug retention time in the skin, and reduce systemic toxicity in the body. These new dosage form drugs will be filed for patent protection prior to entering clinical trials.
-
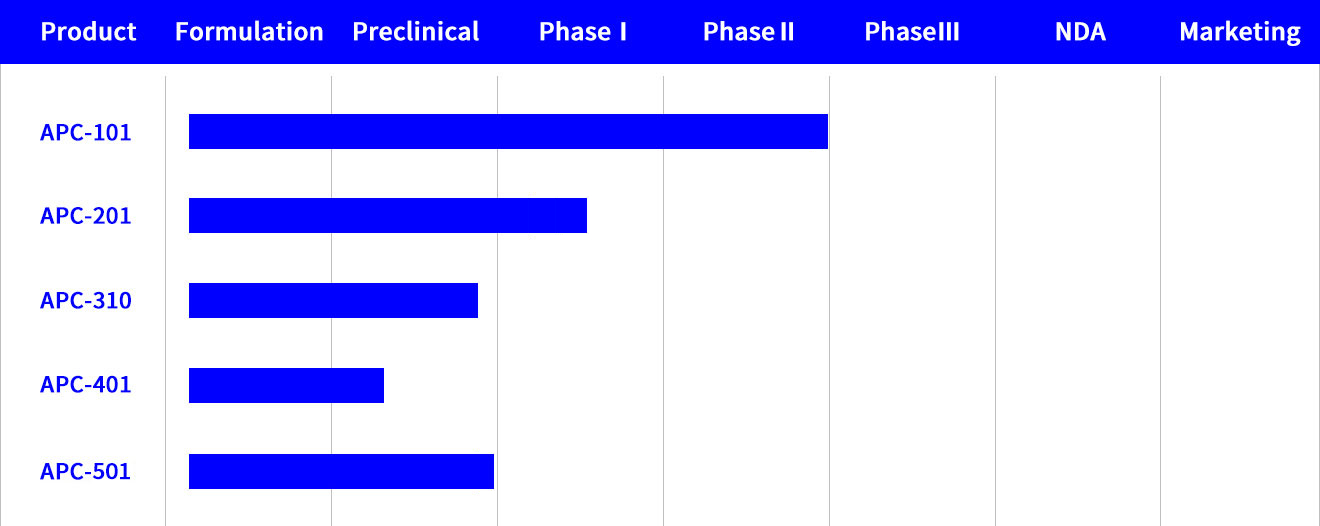
APC101
A fast-drying, film-forming, and meter-dosed spray was developed to treat pain associated with post-herpetic neuralgia (PHN). This product shows fast skin permeation and low skin irritation. A special polymer was added in the proprietary formulation of the 5% PHN spray to form a thin film onto the skin when the product is applied. The drug administered from the metered-dose spray can penetrate through the stratum corneum and be retained in the epidermis layers, acting as a reservoir, for sustained release over 24 hours.
APC101 offers the following advantages
- Less adverse reactions such as skin rash and redness at the application sites.
- Contact–free, eliminating pain on skin
- Can be administered twice daily as needed.
- Suitable to uneven skin of PHN patient
- Can be applied to the area above the hairline.
- Faster onset time comparing to current patch product.
Phase I clinical trial of the product was approved by US FDA and was completed in Taiwan. Phase IIa clinical trial was completed in Australia in 2020.
-
APC201
A novel dosage form of topical anesthetic (4% solution) for treatment of Osteoarthritis (OA).Formulated with a proprietary combination of lipid- humectant, this product shows better skin permeation and lower skin irritation than marketed products indicated for OA pain. A Phase I/II study is on going in Australia.
-
APC310
Utilizing Andros’ core technology in lipid-based delivery, the active ingredient (5%) is encapsulated in lipospheres. The product, indicated for anesthesia for intravenous catheter placement and venipuncture, aims at shortening the onset time and allows convenient storage conditions in ambient temperature. The product is currently in preclinical phase.
-
APC401
The product (5%) is a special emulsified solution, indicated for anesthesia of post-surgery pain. The product is developed to prolong drug retention time in the skin to achieve 3-day long term anesthesia. This product is currently in preclinical phase.
-
APC501
This 14% product, combination of two local anesthetics formulated with a clay-solvent complex aimed at shortening the onset time while maintaining comparable drug product shelf-life under “ambient” storage conditions. indicated for anesthesia for intravenous catheter placement and venipuncture. This product is currently in preclinical phase.
 |

